LIDE specializes in preclinical studies, offering traditional R&D in-vitro and in-vivo services but recommend a better R&D roadmap for potential drug candidates - we call this platform Functional Diagnosis:
Functional Diagnosis Study Roadmap:
- Use conditionally reprogrammed (CR) cells instead of genetically engineered cell lines for in-vitro studies, providing a more representative starting point that has maintained cell heterogeneity.
- Testing or validating indications by leveraging our MiniPDX before PDX studies, a faster, more cost-effective feedback mechanism before more costly investments.
- Running traditional, gold-standard PDX studies to support IND candidates.
- Applying OMICS analysis and MiniPDX® with fresh patient samples to identify biomarkers to inform patient stratification and recruitment strategy
Benefits of Functional Diagnosis:
- Better quality testing with CR cells and PDX models derived from fresh patient samples
- Earlier, cheaper and faster in-vivo validation with MiniPDX® 7-day drug screen
- Increased likelihood of success in clinical trials with OMICS analysis
Functional Diagnosis leverages LIDE's Translational Medicine platform. We are participating in a real-world study that offers cancer patients personalized treatment recommendations. This has helped LIDE receive models of rare cancer types, hard-to-find genetic mutations and drug resistant tissues, with 1700+ PDX models and many high profile expression phenotypes.
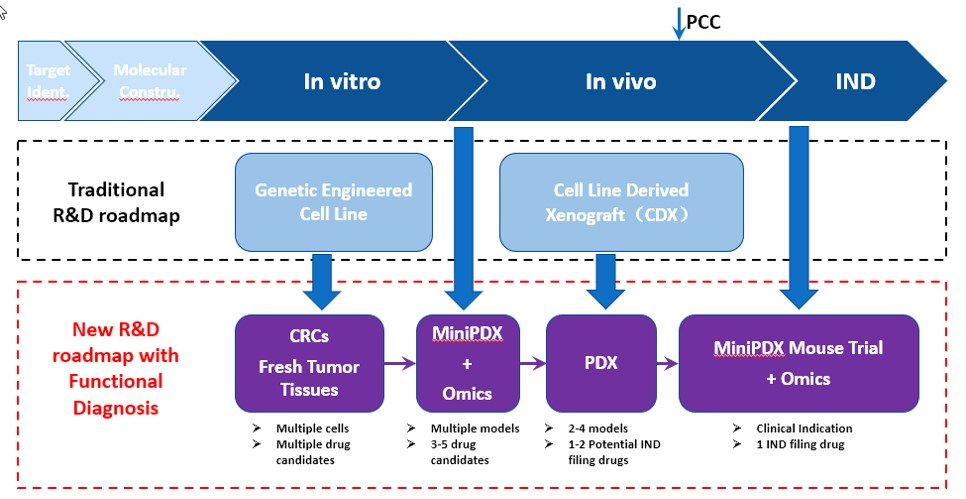
Fig. New, optimized R&D roadmap using LIDE Functional Diagnosis platform